Abstract
Background: The clinical course of follicular lymphoma (FL) is characterized by a slow progression over years with continuous relapses despite good response to initial treatment. The median overall survival is 10 to more than 15 years. Standard therapy for patients requiring treatment consists of an anti-CD 20 antibody combined with chemotherapy followed by antibody maintenance. With this combination a 1-year-PFS of 93% was seen in the GLSG-2000 trial (Hiddemann et al, Blood 2005). Because of the substantial side effects of chemotherapy such as infections, secondary malignancies and impairment of the stem cell reserve novel "chemotherapy-free" treatment approaches could substantially improve the treatment tolerability in FL. The BTK-inhibitor ibrutinib has demonstrated promising activity in patients with iNHL, CLL and MCL. Anticipating the recent reports on a superior activity of obinutuzumab as compared to rituximab in the GALLIUM trial (Marcus et al., NEJM 2017), the GLSG initiated a phase II study combining ibrutinib and obinutuzumab to explore the efficacy and safety of this "chemotherapy-free" alternative.
Methods: ALTERNATIVE is a prospective multicenter single-arm phase 2 study of the combination of ibrutinib and obinutuzumab in 98 patients with previously untreated FL and a high tumor burden (defined by modified GELF criteria) in need of treatment. Induction comprises 6 cycles of obinutuzumab at a dose of 1000 mg by intravenous infusion on days 1, 8, 15 of cycle 1 and on day 1 of cycles 2-6 to be given every 21 days. Ibrutinib is administered orally at a dose of 560 mg once daily throughout all 6 cycles. In patients with at least partial response (defined by Cheson Response Criteria 2007) after the end of induction, maintenance with obinutuzumab (1000mg every 8 weeks) plus ibrutinib (560mg daily) is given for an additional 24 months. In patients remaining MRD positive at 30 months ibrutinib is continued for another 12 months in an extended maintenance setting without obinutuzumab. The primary efficacy endpoint is the rate of investigator-assessed PFS one year after registration. Response rates at end of induction, after one year and after end of maintenance, duration of response, percentage of progression during induction and maintenance, time to treatment failure, overall survival, duration of molecular remission in MRD negative patients and safety are key secondary endpoints.
Results: 98 patients with advanced stage FL were included, The median age was 59 years (29-81), 60% were male and 40% had a high risk FLIPI, 90% stage III/IV disease and 10% were stage II with a high tumor burden.
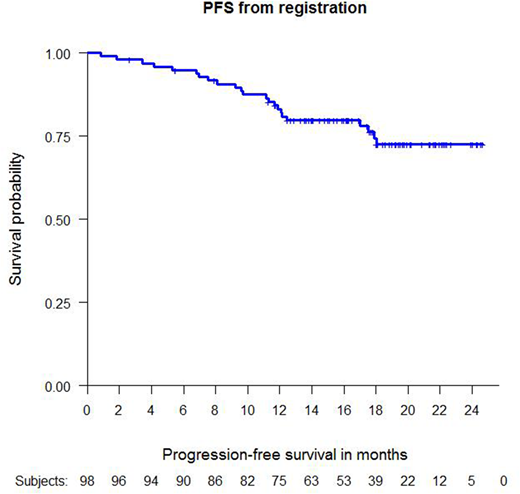
Response to in induction was 90% (87/97) with 85% (82/97) PR and 5% (5/97) CR. 5 patients (5%) progressed during induction. Of the 82 patients with PR after end of induction, 8 patients achieved a CR during the first 6 months of maintenance treatment. 95 patients were evaluable for the primary endpoint of 1-year-PFS and 76 patients (80%) remained alive and free of progression at this timepoint. 18 patients progressed in the first year, two of whom died due to progressive disease. One additional death was caused by a non-lymphoma related event.
An MRD-marker was found in 65 patients. MRD at the end of induction was evaluable for 63 patients. 44 patients (70%) were MRD negative after induction treatment. Of the 42 patients with follow-up MRD peripheral blood or bone marrow samples, 35 (83%) were MRD negative one year after registration.
Therapy was generally well tolerated. Most common adverse events were diarrhea in 30% of patients, rash in 25% and fatigue and nasopharyngitis (common cold) in 23% and 20%, respectively. Concerning hematotoxicity grade 3-4 neutropenia and thrombopenia were seen in 8% and 4% of patients, respectively. Severe (>=grade 3) infectious complications were rare (6% pneumonia/bronchitis, 2% sepsis, 7% other infections).
Conclusions: The chemotherapy - free combination of ibrutinib and obinutuzumab showed high anti-lymyphoma activity with high overall response rates and a high proportion of MRD negativity at one year. While the combination of ibrutinib and obinutuzumab was associated with a low toxicity profile, the combination was inferior to the published results of conventional immunochemotherapies in terms of the primary efficacy endpoint (1-year-PFS).
Further evaluations might demonstrate whether subgroups exist which particularly benefit clinically from this low toxicity regime.
Schmidt:Celgene: Honoraria; Gilead: Honoraria, Other: Travel Grants; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants. Buske:Bayer: Research Funding; Roche: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Viardot:Amgen: Consultancy; Gilead Kite: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Keller:BMS: Consultancy; Roche: Consultancy; Takeda: Consultancy, Research Funding; Janssen-Cilag: Consultancy, Equity Ownership; MSD: Consultancy; Celgene: Research Funding. Graeven:Roche: Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria. Marks:Merck: Honoraria; BMS: Honoraria; Servier: Honoraria. Hänel:Novartis: Honoraria; Roche: Honoraria; Amgen: Honoraria; Takeda: Honoraria. Liersch:Roche: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria. Dürig:Celgene: Honoraria; Roche: Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria. Hoster:Roche Pharma AG: Other: Travel support, Research Funding; F. Hoffman-La Roche: Other: Travel support, Research Funding. Unterhalt:F. Hoffman-La Roche: Other: Travel support. Hiddemann:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffman-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal